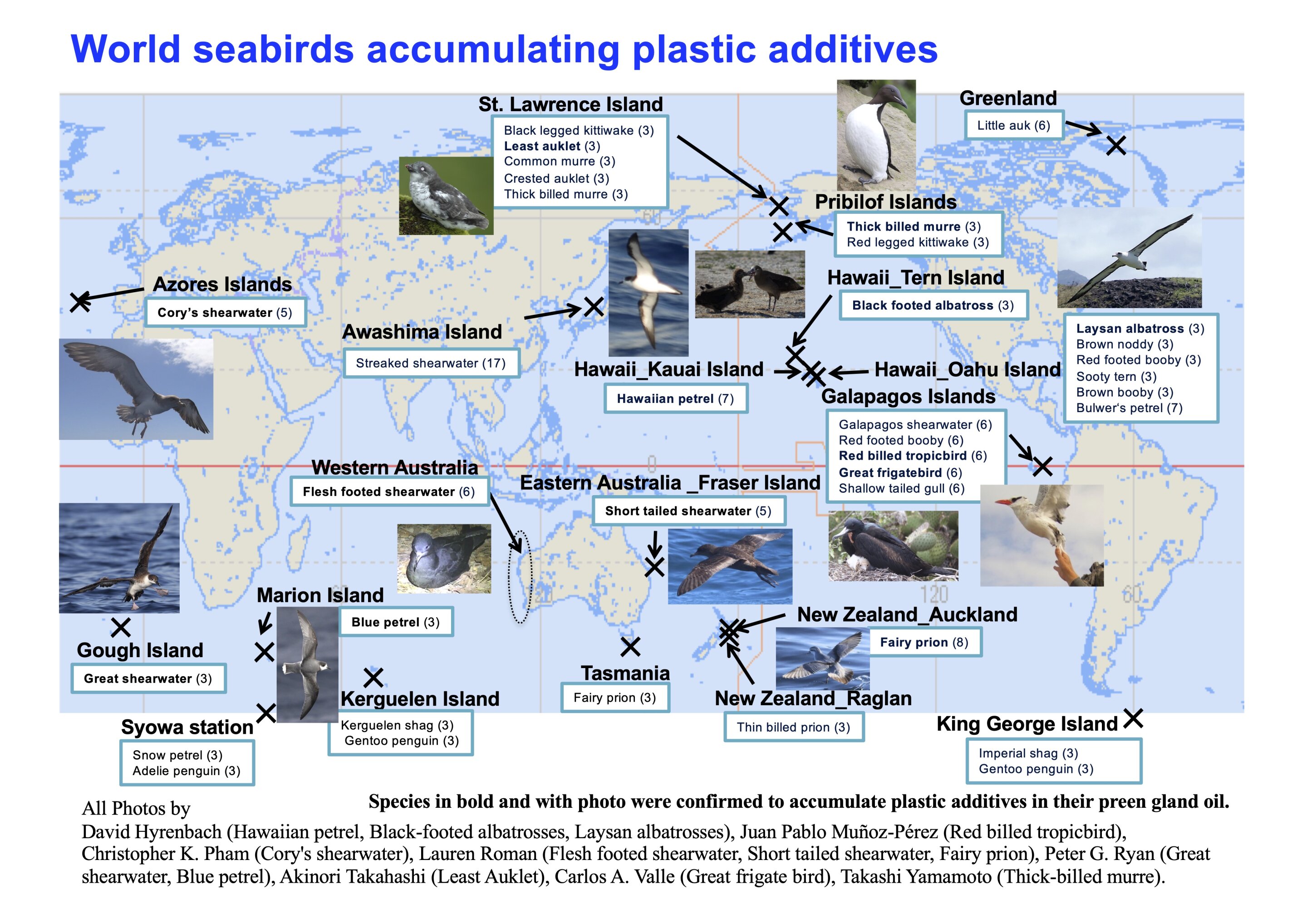
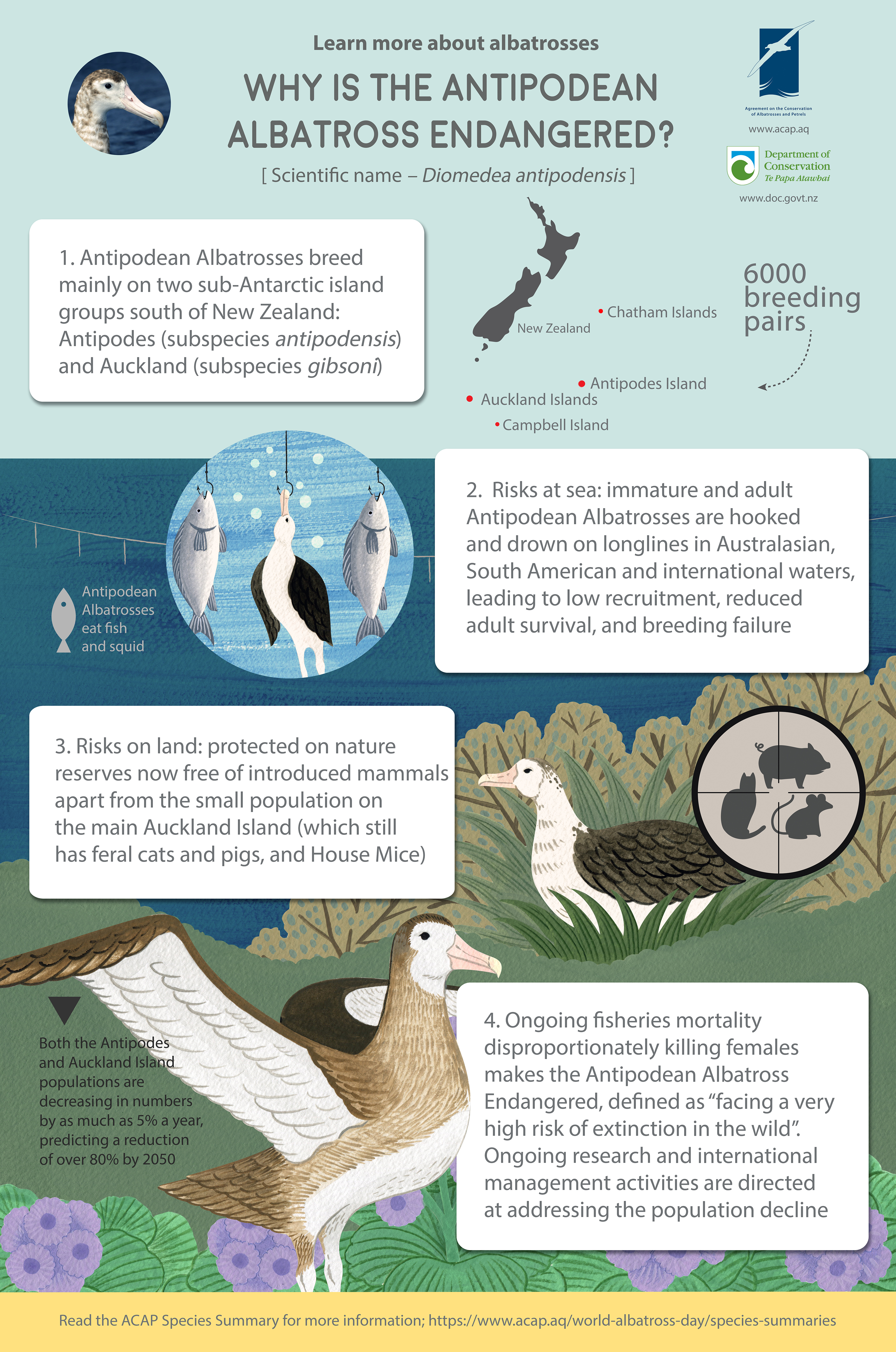
On ‘Macca’ Light-mantled Albatrosses breed among sub-Antarctic megaherbs, here the broad-leafed Macquarie Island Cabbage Stilbocarpa polaris
NOTE: This post continues an occasional series that features photographs of the 31 ACAP-listed species, along with information from and about their photographers. Here, Jaimie Cleeland, a Fisheries Scientist at the Australian Antarctic Division and the University of Tasmania, describes her research conducted on the globally Near Threatened Light-mantled Albatrosses Phoebetria palpebrata that breed on Australia’s sub-Antarctic Macquarie Island.

Jaimie Cleeland approaches Light-mantled Albatrosses breeding on the steep slopes of Macquarie Island; all such visits are conducted under a research permit
If you’re ever lucky enough to visit one of the many sub-Antarctic islands that Light-mantled Albatrosses breed on – you will mostly likely hear them before you see them! Their Sky Point display, coupled with a distinctive – and evocative - “pee-aahh” call, is what first caught my attention and drew my eyes to the steep escarpments of Macquarie Island. It is on these exposed cliffs that the Light-mantled Albatrosses breed – making it challenging for field biologists, such as myself, to access their nests to check their breeding status or read the number of a leg band. Although it doesn’t usually take very long – perhaps just a few visits to the monitoring colony before you achieve “mountain goat” abilities and become comfortable working safely at heights. It is then your attention can turn to their aerial acrobatics as pairs whizz by in synchronized flight – a display of courtship.

The light blue sulcus on the lower mandible distinguishes the Light-mantled from the Sooty Albatross with its yellow sulcus
During the Macquarie Island Pest Eradication Project (MIPEP), I regularly visited several of the island’s research monitoring sites to conduct breeding surveys of Light-mantled Albatrosses from 2011 to 2014 (click here). In 2013, with the help of a dedicated team of rabbit and rodent hunters, we scoured the whole island, finding 2151 occupied nests. A ground search of this magnitude, conducted over steep terrain, was a huge achievement for our team.

A Light-mantled Albatross chick sits up to eye the photographer
Light-mantled Albatrosses then became a subject of my PhD thesis at the University of Tasmania. Even though I was no longer living on “Macca”, as the island is affectionally called by its human visitors, I spent my days trying to understand their patterns in foraging behaviour and their vulnerability to invasive species, climate change and fisheries impacts. I found that during breeding, Light-mantled Albatrosses foraged farther south than any of the other albatrosses that breed on Macquarie and during the non-breeding period some tracked individuals even circumnavigated the whole of Antarctica.

Light-mantled Albatrosses gather to meet and greet
Like all albatrosses, Light-mantled Albatrosses spend the majority of their life at sea. For the birds breeding on Macquarie I found large-scale climate cycles such as the El Nino – Southern Oscillation (ENSO) and the Southern Annular Mode influenced their survival. Despite their predominantly oceanic life. I also found conditions at the colony can impact this species. Damage to nesting habitat on the steep slopes of Macquarie Island caused by heavy rabbit grazing reduced their likelihood of breeding.

A Light-mantled Albatross fly by
Luckily the Macquarie Island Pest Eradication Project was successful in removing rabbits, rats and mice from the island and the slopes that the Light-mantled Albatross call home are lush and green once more!
Selected Publications:
Beal, M., Dias, M.P., Phillips, R.A., Oppel, S., Hazin, C., Pearmain, E.J., Adams, J. , Anderson, D.J., Antolos, M., Arata, J.A., Arcos, J.M., Arnould, J.P., Awkerman, J., Bell, E., Bell, M. Carey, M., Carle, R., Clay, T.A., Cleeland, J., Colodro, V., Conners, M. Cruz-Flores, M., Cuthbert, R., Delord, K., Deppe, L., Dilley, B.J., Dinis, H., Elliott, G., De Felipe, F., J. Felis, M.G. Forero, A. Freeman, A. Fukuda, J. González-Solís, J.P. Granadeiro, A. Hedd, P. Hodum, J. M. Igual, A. Jaeger, T.J. Landers, M. Le Corre, A. Makhado, B. Metzger, T. Militão, W.A. Montevecchi, V. Morera-Pujol, L. Navarro-Herrero, D. Nel, D. Nicholls, D. Oro, R. Ouni, K. Ozaki, F. Quintana, R. Ramos, T. Reid, J.M. Reyes-González, C. Robertson, G. Robertson, M.S. Romdhane, P.G. Ryan, P. Sagar, F. Sato, S. Schoombie, R.P. Scofield, S.A. Shaffer, N.J. Shah, K.L. Stevens, C. Surman, R.M. Suryan, A. Takahashi, V. Tatayah, G. Taylor, D.R. Thompson, L. Torres, K. Walker, R. Wanless, S.M. Waugh, H. Weimerskirch, T. Yamamoto, Z. Zajkova, L. Zango & P. Catry 2021. Global political responsibility for the conservation of albatrosses and large petrels. Science Advances (10). DOI: 10.1126/sciadv.abd7225. [click here].
Carneiro, A.P.B., Pearmain, E.J., Oppel, S., Clay, T.A., Phillips, R.A., Bonnet-Lebrun, A.-S., Wanless, R.M., Abraham, E., Richard, Y., Rice, J., Handley, J., Davies, T.E., Dilley, B.J., Ryan, P.G., Small, C., Arata, J., Arnould, J.P.Y., Bell, E., Bugoni, L., Campioni, L., Catry, P., Cleeland, J., Deppe, L., Elliott, G., Freeman, A., González-Solís, J., Granadeiro, J.P. Grémillet, D., Landers, T.J., Makhado, A., Nel, D., Nicholls, D.G., Rexer-Huber, K., Robertson, C.J.R., Sagar, P.M., Scofield, P., Stahl, J.-C., Stanworth, A., Stevens, K.L., Trathan, P.N., Thompson, D.R., Torres, L., Walker, K., Waugh, S.M., Weimerskirch, H. & Dias, M.P. 2020. A framework for mapping the distribution of seabirds by integrating tracking, demography and phenology. Journal of Applied Ecology doi.org/10.1111/1365-2664.13568. [click here]
Cleeland, J. 2017. Factors that drive demographic change in a community of albatrosses. PhD thesis. Hobart: University of Tasmania. 153 pp. [click here]
Cleeland, J.B., Alderman, R., Bindoff, A., Lea, M.-A., McMahon, C.R., Phillips, R.A., Raymond, B., Sumner, M.D., Terauds, A., Wotherspoon, S.J. & Hindell, M.A. 2019. Factors influencing the habitat use of sympatric albatrosses from Macquarie Island. Marine Ecology Progress Series 609: 221-237. [click here]
Cleeland, J.B., Pardo, D., Raymond, B., Terauds, A., Alderman, R., McMahon, C.R., Phillips, R.A., Lea, M.-A. & Hindell, M.A. 2020. Introduced species and extreme weather as key drivers of reproductive output in three sympatric albatrosses. Scientific Reports: 10: 8199. doi.org/10.1038/s41598-020-64662-5. [click here]
Cleeland, J.B., Pardo, D., Raymond, B., Tuck, G.N., McMahon, C.R., Phillips, R.A., Alderman, R., Lea, M.-A. & Hindell, M.A. 2021. Disentangling the influence of three major threats on the demography of an albatross community. Frontiers in Marine Science doi: 10.3389/fmars.2021.578144. [click here]
Jones, C.W., Risi, M.M., Cleeland, J. & Ryan, P.G. 2019. First evidence of mouse attacks on adult albatrosses and petrels breeding on sub-Antarctic Marion and Gough Islands. Polar Biology doi.org/10.1007/s00300-018-02444-6. [click here].
Requena, S., Oppel, S., Bond, A.L., Hall, A., Cleeland, J., Crawford, R.J.M., Davies, D., Dilley, B.J., Makhado, A., Ratcliffe, N., Reid, T.A., Ronconi, R.A., Schofield, A., Steinfurth, A., Wege, M., Bester, M.[N.] & Ryan, P.G. 2020. Marine hotspots of activity inform protection of a threatened community of pelagic species in a large oceanic jurisdiction. Animal Conservation doi.org/10.1111/acv.12572. [click here]
Jaimie Cleeland, Australian Antarctic Division, Kingston and University of Tasmania, Hobart, Australia, 15 October 2021

 English
English  Français
Français  Español
Español